DEPENDABLE SOLUTIONS FOR COMPLEX CASES
Clinical Evidence RESTOR-1 Study1
AngioSafe’s Atheroplasty™ platform is backed by rigorous clinical evaluation. Designed to enable safe, efficient, and wire-free crossing of complex CTOs.
RESTOR-1 Pivotal Trial:
A prospective, multi center, pivotal study, conducted at 14 sites in the United States with 74 patients enrolled, treated, and core lab adjudicated.
Objective:
To demonstrate the safety and effectiveness of the Santreva™-ATK System to facilitate crossing of CTOs in the peripheral vasculature.
Key Inclusion Criteria:
- Rutherford Classification Criteria 2-5
- Femoropopliteal arteries
- 99-100% stenosed
- Max 300mm long, regardless of calcium severity
Primary Endpoint:
Clinical success of Santreva-ATK to facilitate placement of guidewire into the distal true lumen of the femoropopliteal artery CTO, in the absence of device-related major adverse events (MAE) through discharge or 24 hours post-procedure, whichever is sooner.
AngioSafe delivers compelling
results where it matters most.
or bailout stent
time (min)
time (min)
length (mm)
Built to perform where patient and
lesion complexity converge
crossed antegrade only
was completed intraluminal
a 2.8mm channel in a 5mm vessel, representing >55% mean lumen gain in femoropopliteal arteries. Data on file.
In a single pass, Santreva ATK successfully crossed even the most complex lesions, irrespective of level of calcification or lesion length
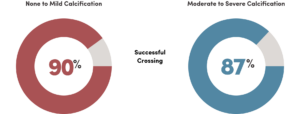
Traditional CTO procedures rely on multiple devices to cross, then open the vessel. Our technology merges those steps delivering a more efficient, streamlined experience with the potential to reduce complications, reduce procedure times, and improve outcomes.
Significant Improvement in Rutherford Classification and Pain Scores
From proximal cap to true lumen
Introducing Santreva-ATK
Santreva-ATK is an all-in-one, large-profile, manually controlled, and guidewire-free endovascular CTO crossing solution, with full force range and control to tackle the wide range of plaque morphologies, designed to limit damage to the vessel wall.
1 RESTOR-1 Study, data on file (ClinicalTrials.gov ID: NCT04663867)